| Sandoz | PrSandoz® Dutasteride | |  | 02424444 |
| Sandoz | Sandoz® Desvenlafaxine | |  | 02538415 |
| Sandoz | Sandoz Rivastigmine Patch | |  | 02426293 |
| Sandoz | Sandoz® Bisoprolol | |  | 02544245 |
| Sandoz | Sandoz Lisdexamfetamine | |  | 02546248 |
| Pharmascience | pms-TRAZODONE | |  | 01937227 |
| Sandoz | Sandoz® Gliclazide MR and Sandoz® Pravastatin | |  | 02461323 |
| Sandoz | Sandoz Docusate Sodium | |  | |
| Pharmascience | pms-PERINDOPRIL 8mg | |  | 02470691 |
| Pharmascience | pms-FLUOXETINE 20mg CAPS | |  | 02177587 |
| Pharmascience | PERICHLOR 0.12% ORAL RINSE | |  | 02493160 |
| Pharmascience | | None Selected | | |
| Sandoz | | None Selected | | |
| Pharmascience | pms-ROSUVASTATIN 5mg TABS 3000 | | | 02378523 |
| Sandoz | Sandoz Gliclazide MR | |  | 02314177 |
| Sandoz | NYPOZI | |  | 02520990 |
| Sandoz | NYPOZI - ZIEXTENZO | |  | 02520990 |
| Pharmascience | | None Selected | | |
| Pharmascience | pms-DICLOFENAC 1.5% SOL TOPI 60 mL | |  | 02356783 |
| Pharmascience | pms-METHOTREXATE 50MG/ML | |  | 2539608 |
| Pharmascience | pms-PERINDOPRIL-AMLODIPINE | |  | 02541696 |
| Pharmascience | pms-CITALOPRAM HBR 40mg TAB 1100, | |  | 02248011 |
| Sandoz | Sandoz Apremilast | |  | 02529092 |
| Sandoz | Sandoz Omeprazole | |  | 02296438 |
| Pharmascience | pms-PREGABALIN 150mg 800 | | | 2359634 |
| Pharmascience | pms-LACTULOSE-PHARMA 667mg/mL | |  | 02247383 |
| Pharmascience | pms-QUINAPRIL 40mg TABS 100 | |  | 02340585 |
| Pharmascience | SILDENAFIL 100mg | |  | 02317583 |
| Pharmascience | pms-TERAZOSIN 2mg TABS 100 | |  | 02243519 |
| Pharmascience | pms-LITHIUM CARBONATE 300mg CAPS 100 | |  | 02216140 |
| Pharmascience | pms-PAZOPANIB | New Product | Nouveau produit |  | 02525666 |
| Pharmascience | pms-MONTELUKAST CHEW | New pack size | Nouveau format |  | 02354977 |
| Sandoz | Amlodipine 2.5 mg | New pack size | Nouveau format |  | 02330474 |
| Pharmascience | PHARMASCIENCE NEW XL FORMATS | New pack size | Nouveau format |  | 02244840, 02359596, 02359618, 02240606 |
| Pharmascience | PHARMASCIENCE NEW XL FORMATS | New pack size | Nouveau format |  | 02248010, 02470683, 02359626 |
| Pharmascience | pms-DIGOXIN 0.05mg/mL ORAL SOL 115mL | Product Transition | Transition dans la production |  | 02242320 |
| Pharmascience | | None Selected | | 02533332 |
| Pharmascience | pms-CIPROFLOXACIN XL | Product Availability | Disponibilité des produits |  | 2416433 |
| Pharmascience | OLESTYR REGULAR & LIGHT POWDER (ORANGE) | Important Announcement | Annonce importante | | 02210320, 00890960 |
| Pharmascience | Perindopril-Indapamide | New Product | Nouveau produit |  | 02537990,02538008,02537982 |
| Sandoz | Sandoz Diclofenac SR | Discontinuation | Abandon de produit |  | 02261944 |
| Pharmascience | pmsc-METFORMIN 500mg and 850mg | Product Appearance Up date | Nouvel aspect du produit |  | 02520303, 02520311 |
| Sandoz | Sandoz® Losartan and Sandoz® Losartan HCT | New pack size | Nouveau format |  | 02313340, 02313359, 02313375, 02362449, 02313383 |
| Pharmascience | pms-RAMIPRIL-HCTZ 10/25mg TABS | Product Transition | Transition dans la production |  | 02342170 |
| Pharmascience | TOLOXIN 0.0625mg & 0.125ng TABS 250 to pms-DIGOXIN 0.0625mg & 0.125mg TABS 250 | Product Transition | Transition dans la production |  | 02335700 & 02335719 |
| Sandoz | Pr Daptomycin for injection | New pack size | Nouveau format |  | 02490838 |
| Sandoz | Pr Gemcitabine injection | New pack size | Nouveau format |  | 02412292 |
| Pharmascience | pms-FLUTICASONE HFA 50mcg/ACT 120 | New Product | Nouveau produit |  | 02503115, 02503123, 02503131 |
| Pharmascience | pms-QUINAPRIL 5mg, 10mg and 20mg TABS 100 | Product Availability | Disponibilité des produits |  | 02340550, 02340569, 02340577 |
| Sandoz | Sandoz Sunitinib | New Product | Nouveau produit |  | NA |
| Sandoz | Sandoz Dapagliflozin | New Product | Nouveau produit |  | NA |
| Pharmascience | pms-Dapagliflozin | New Product | Nouveau produit |  | 0231550,0231569 |
| Sandoz | PrClindamycin Injection USP-60ml | Product Availability | Disponibilité des produits |  | 02230535 |
| Pharmascience | pms-CLARITHROMYCIN 250mg & 500mg Tabs | Discontinuation | Abandon de produit |  | 02247573, 02247574 |
| Sandoz | Sandoz Alfacalcidol | New Product | Nouveau produit |  | 02533316,02533324 |
| Pharmascience | pms-PIRFENIDONE | New Product | Nouveau produit |  | 02531526, 02531534 |
| Pharmascience | METHOTREXATE Injection PFS 25mg/mL | New Product | Nouveau produit |  | 02422204 |
| Sandoz | Sandoz Indomethacin & Sandoz Prochlorperazine | Important Announcement | Annonce importante |  | 02231799,02231800,00789720 |
| Sandoz | Sandoz Montelukast | Product Appearance Up date | Nouvel aspect du produit |  | 02328593 |
| Pharmascience | pmsc-ESOMEPRAZOLE DR | New Product | Nouveau produit |  | 02528479,02528487 |
| Pharmascience | OCTASA 1600 mg, mesalamine delayed-release oral tablets | New Product | Nouveau produit |  | 02529610 |
| Pharmascience | pms-ATOMOXETINE 10mg TABS | Product Transition | Transition dans la production |  | 02381028 |
| Pharmascience | pms-MONTELUKAST CHEW 4mg TABS 100 | Product Transition | Transition dans la production |  | 02354977 |
| Pharmascience | pms-MONTELUKAST CHEW 5mg TABS BLI 3X10 | Product Availability | Disponibilité des produits |  | 02354985 |
| Mylan | Dymista | Information Update | Mise à jour des renseignements |  | 02432889 |
| Pharmascience | pms-BETAHISTINE | Product Availability | Disponibilité des produits |  | 02330210, 02330237 |
| Pharmascience | pmsc-VENLAFAXINE XR | Product Availability | Disponibilité des produits |  | 02521466,02521482,02521474 |
| Pharmascience | pms-CINACALCET | New Product | Nouveau produit |  | 02517604,02517612,02517620 |
| Sandoz | Sandoz Sitagliptin | New Product | Nouveau produit |  | NA |
| Sandoz | Sandoz Sitagliptin-Metformin | New Product | Nouveau produit |  | NA |
| Teva | TEVA-CEFADROXIL, TEVA-TOPISONE (CREAM), TEVA-ALMOTRIPTAN | Formulary Update | Mise à jour du formulaire |  | 02235134, 00804991, 02434849 |
| Teva | TEVA-TOPISONE (CREAM) | Formulary Update | Mise à jour du formulaire |  | 00804991 |
| Teva | Teva-Diltiazem XC | New Product | Nouveau produit | 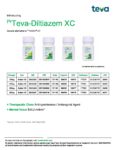 | 02429322, 02429330, 02429349, 02429357 |
| Apotex | pms-QUETIAPINE 200mg TABS 500 | Product Transition | Transition dans la production |  | 00703486 |
| Apotex | pms-CLARITHROMYCIN 500mg TABS 250 | Product Transition | Transition dans la production |  | 02278561 |
| Teva | Teva-Diltiazem XC | New Product | Nouveau produit | 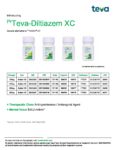 | 02429322, 02429330, 02429349, 02429357 |
| Teva | | Formulary Update | Mise à jour du formulaire | | 345345 |
| Sandoz | Supeudol | Product Availability | Disponibilité des produits |  | 00392480,00392472 |
| Sandoz | Sandoz Prochlorperazine Suppository | Product Availability | Disponibilité des produits |  | 00789720 |
| Pharmascience | pms-METHOTREXATE 2.5mg bottles of 100's | New Product | Nouveau produit | 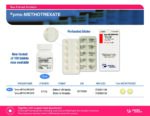 | 02170698 |
| Apotex | KleanLyte | Product Availability | Disponibilité des produits |  | 80107241 |
| Sandoz | Sandoz Ambrisentan | New Product | Nouveau produit |  | 0000 |
| Sandoz | Sandoz Mycophenolate Mofetil | Product Appearance Up date | Nouvel aspect du produit |  | 02320630,02313855 |
| Apotex | pms-IRBESARTAN 150mg 500 | Product Transition | Transition dans la production |  | 02317079 |
| Teva | Teva-Valsartan | Product Availability | Disponibilité des produits |  | 02356678 |
| Teva | Teva-Pregabalin | Product Availability | Disponibilité des produits |  | 02361159, 02361175, 02361183, 02361205, 02361221, 02361248 |
| Teva | TEVA-TERIFLUNOMIDE | Formulary Update | Mise à jour du formulaire |  | 02501090 |
| Teva | | Formulary Update | Mise à jour du formulaire | | 02501090 |
| Teva | TEVA-NAPROXEN SODIUM, TEVA-NAPROXEN SODIUM DS | Formulary Update | Mise à jour du formulaire |  | 00778389, 02026600 |
| Teva | Teva-Teriflunomide | Formulary Update | Mise à jour du formulaire |  | 02501090 |
| Pharmascience | pharma-SIMVASTATIN 20mg TABS 500 | Product Transition | Transition dans la production |  | 02469995 |
| Pharmascience | pms-ABACAVIR/LAMIVUDINE600/300mg T 3X10 | Product Availability | Disponibilité des produits |  | 02458381 |
| Teva | TEVA-FLUTICASONE | Formulary Update | Mise à jour du formulaire |  | 02453738 |
| Teva | TEVA-TERIFLUNOMIDE, TEVA-DESVENLAFAXINE | Formulary Update | Mise à jour du formulaire |  | 02501090, 02458217, 02458225 |
| Pharmascience | pms-DEFERASIROX FC (Type J) | New Product | Nouveau produit |  | 02528290/02528312 |
| Teva | Teva-Pregabalin | Product Availability | Disponibilité des produits |  | 02361159, 02361175, 02361183, 02361205, 02361221, 02361248 |
| Pharmascience | pms-SILDENAFIL R 20mg TABS BLI 6X15 | Product Transition | Transition dans la production |  | 02412179 |
| Mylan | FULPHILA ONE CERT | Important Announcement | Annonce importante |  | 02484153 |
| Teva | Teva-Pregabalin | Product Availability | Disponibilité des produits |  | 02361159, 02361175, 02361183, 02361205, 02361221, 02361248 |
| Teva | Aermony RespiClick | Information Update | Mise à jour des renseignements |  | 02467895, 02467909, 02467917 |
| Pharmascience | pms-TERIFLUNOMIDE 14MG | Formulary Update | Mise à jour du formulaire |  | 02500434 |
| Teva | TEVA-TERIFLUNOMIDE | Formulary Update | Mise à jour du formulaire |  | 02501090 |
| Teva | TEVA-TERIFLUNOMIDE | Formulary Update | Mise à jour du formulaire |  | 02501090 |
| Teva | Teva-Pregabalin | Product Availability | Disponibilité des produits |  | 02361159, 02361175, 02361175, 02361221, 02361248 |
| Teva | REVIA | Formulary Update | Mise à jour du formulaire |  | 02213826 |
| Pharmascience | pms-ATORVASTATIN 10mg - 20mg - 40mg | Product Transition | Transition dans la production |  | 02399377-02399385-02399393 |
| Pharmascience | pmsc-METFORMIN | New pack size | Nouveau format |  | 02520303 |
| Pharmascience | pms-CELECOXIB 200mg | Product Transition | Transition dans la production |  | 02355450 |
| Sandoz | Sandoz Capsule June 2022 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule June 2022 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule June 2022 | Important Announcement | Annonce importante |  | 0000 |
| Apotex | New Product Launch: Apo-Apixaban 2.5 mg & 5 mg Tabs | New Product | Nouveau produit |  | 02487381, 02487403 |
| Teva | Teva-Teriflunomide | New Product | Nouveau produit |  | 02501090 |
| Teva | TEVA-TERIFLUNOMIDE | Formulary Update | Mise à jour du formulaire |  | 02501090 |
| Sandoz | Milrinone Lactate Injection | Discontinuation | Abandon de produit |  | 02244854 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Sandoz | Sandoz Capsule May 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule May 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule May 2022 | Important Announcement | Annonce importante |  | 0000 |
| Teva | Teva-Teriflunomide | New Product | Nouveau produit |  | 02501090 |
| Apotex | Ontario pan-Canadian Price Change: Effective May 31, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Pharmascience | pms-ABACAVIR/LAMIVUDINE 600/300mg TAB 30 | Product Availability | Disponibilité des produits |  | 02458381 |
| Sandoz | Acetylcysteine Solution | Product Availability | Disponibilité des produits |  | 02243098 |
| Teva | Teva-Teriflunomide | New Product | Nouveau produit |  | 02501090 |
| Apotex | New Product Launch: Apo-Teriflunomide 14 mg Tabs | New Product | Nouveau produit |  | 02500639 |
| Sandoz | Sandoz Teriflunomide | New Product | Nouveau produit |  | 0000 |
| Pharmascience | pms-Teriflunomide | New Product | Nouveau produit |  | 02500434 |
| Sandoz | Methylphenidate SR | Discontinuation | Abandon de produit |  | 02320312 |
| Sandoz | Topiramate Tab | Discontinuation | Abandon de produit |  | 02431807, 02431815, 02431823 |
| Pharmascience | pmsc-METFORMIN | New pack size | Nouveau format |  | 02520303 |
| Apotex | Manitoba pan-Canadian Price Change: Effective June 1, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Nova Scotia Formulary Update: Effective May 9, 2022 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Saskatchewan Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Mylan | Introducing ABEVMY | New Product | Nouveau produit |  | 02522160, 02522179 |
| Pharmascience | pms-VALPROIC ACID E.C 500MG TABS | Important Announcement | Annonce importante |  | 02229628 |
| Sandoz | Piperacillin and Tazobactam | New Product | Nouveau produit |  | 02521539 |
| Teva | TEVA-DESVENLAFAXINE | Formulary Update | Mise à jour du formulaire |  | 02458217, 02458225 |
| Sandoz | Sandoz Capsule April 2022 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule April 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule April 2022 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule April 2022 | Important Announcement | Annonce importante |  | 0000 |
| Mylan | Introducing ABEVMY | New Product | Nouveau produit |  | 02522160,02522179 |
| Mylan | ACCESSA and Fulphila | Important Announcement | Annonce importante |  | 02484153 |
| Mylan | ONE CERT for Fulphila | Important Announcement | Annonce importante |  | 02484153 |
| Teva | Teva-Desvenlafaxine | New Product | Nouveau produit | 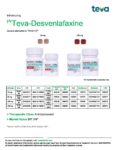 | 02458217, 02458225 |
| Teva | TEVA-VALGANCICLOVIR | Information Update | Mise à jour des renseignements |  | 02413825 |
| Teva | TEVA-DESVENLAFAXINE | Formulary Update | Mise à jour du formulaire |  | 02458217, 02458225 |
| Teva | TEVA-DESVENLAFAXINE | Formulary Update | Mise à jour du formulaire |  | 02458217, 02458225 |
| Apotex | pms-ATOMOXETINE 18mg BLIS 2x15 | Product Transition | Transition dans la production |  | 02381036 |
| Sandoz | Sandoz Lurasidone | New Product | Nouveau produit |  | 0000 |
| Apotex | pms-METFORMIN 850mg | Product Transition | Transition dans la production |  | 02242589 |
| Apotex | pms-METFORMIN 500mg | Product Transition | Transition dans la production |  | 02223562 |
| Apotex | METONIA 5MG TABS 100 & METONIA 1mg/mL SOL 500mL | Product Transition | Transition dans la production |  | 02230431 & 02230433 |
| Teva | TEVA-VALGANCICLOVIR | Information Update | Mise à jour des renseignements |  | 02413825 |
| Pharmascience | pharma-SIMVASTATIN 80MG BLIS | Product Substitution | Substitution de produit |  | 02470012 |
| Pharmascience | PANTOPRAZOLE 40MG | Product Transition | Transition dans la production |  | 02437945 |
| Sandoz | Sandoz Capsule March 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Capsule Sandoz March 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule March 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Finasteride | Product Appearance Up date | Nouvel aspect du produit |  | 02322579 |
| Mylan | FULPHILA ONE CERT Program | Important Announcement | Annonce importante |  | 02484153 |
| Sandoz | Isoproterenol Hydrochloride Injection USP | Product Availability | Disponibilité des produits |  | 00897639 |
| Teva | Teva OTC Allergy Products and First Aid Solutions | Information Update | Mise à jour des renseignements |  | 00021288, 02097583, 02097583, 02097575 |
| Apotex | Manitoba Price Change: Effective April 1, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Teva | TEVA-ROSUVASTATIN | Information Update | Mise à jour des renseignements |  | 02354608, 02354616, 02354624, 02354632 |
| Teva | Teva-Desvenlafaxine | New Product | Nouveau produit | 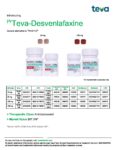 | 02458217, 02458225 |
| Sandoz | FLUOXETINE CAP | Discontinuation | Abandon de produit |  | 02479486, 02479494 |
| Pharmascience | pms-LOSARTAN 25MG,50MG & 100MG | Product Availability | Disponibilité des produits |  | 02309750, 02309769 & 02309777 |
| Apotex | Newfoundland Price Change: Effective March 8, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Sandoz | TRANSDERM-V (SCOPOLAMINE PATCH) | Discontinuation | Abandon de produit |  | 80024336 |
| Pharmascience | pms-RISPERIDONE 3mg TABS BLI 6X10 | Product Transition | Transition dans la production |  | 02252058 |
| Pharmascience | pms-RISPERIDONE 2mg TABS BLI 6X10 | Product Transition | Transition dans la production |  | 02252031 |
| Pharmascience | pms-RISPERIDONE 3mg TABS 500 | Product Transition | Transition dans la production |  | 02252058 |
| Pharmascience | pms-RISPERIDONE 2mg TABS 500 | Product Transition | Transition dans la production |  | 02252031 |
| Pharmascience | pms-LOSARTAN 100mg | Product Availability | Disponibilité des produits |  | 02309777 |
| Apotex | Ontario Price Change: Effective February 28. 2022 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Newfoundland and Labrador Formulary Update: Effective March 1, 2022 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Sandoz | Bortezomib for Injection SDZ | New Product | Nouveau produit |  | 02503352 |
| Apotex | New Brunswick Price Change: Effective February 28, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Sandoz | Comtan Discontinuation | Discontinuation | Abandon de produit |  | 0000 |
| Teva | ACT RIZATRIPTAN | Information Update | Mise à jour des renseignements |  | 02381702 |
| Teva | TEVA-TELMISARTAN HCTZ | Product Availability | Disponibilité des produits |  | 02330288, 02379252 |
| Sandoz | Sandoz Capsule February 2022 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule February 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule February 2022 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule February 2022 | Important Announcement | Annonce importante |  | 0000 |
| Apotex | Alberta Price Change: Effective March 1, 2022 | Price Update | Mise à jour des prix |  | 0 |
| Teva | Teva-Desvenlafaxine | New Product | Nouveau produit | 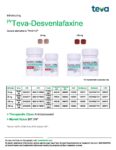 | 02458217, 02458225 |
| Teva | TEVA-ROSUVASTATIN | Information Update | Mise à jour des renseignements |  | 02354608, 02354616, 02354624, 02354632 |
| Teva | Teva-Desvenlafaxine | New Product | Nouveau produit | 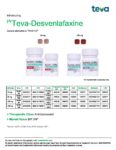 | 02458217, 02458225 |
| Apotex | New Product Launch: Apo-Guanfacine XR 1 mg, 2 mg, 3 mg and 4 mg Tablets | New Product | Nouveau produit |  | 02523728, 02523736, 02523744, 02523752 |
| Sandoz | Sandoz Clonidine | New Product | Nouveau produit |  | 0000 |
| Sandoz | Sandoz Tramadol | Product Transition | Transition dans la production |  | 000 |
| Pharmascience | pms-LOSARTAN 25mg & 50mg | Product Availability | Disponibilité des produits |  | 02309750 & 02309769 |
| Teva | Teva-Telmisartan HCTZ | Product Availability | Disponibilité des produits |  | 02330288, 02379252 |
| Teva | | Formulary Update | Mise à jour du formulaire | | 02230584, 02230585, 02503751, 02503778, 02503786 |
| Apotex | NIHB Formulary Update: Effective Immediately | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Alberta Formulary Update: Effective February 1, 2022 | Formulary Update | Mise à jour du formulaire |  | 02505762 |
| Teva | TEVA-TELMISARTAN HCTZ | Product Availability | Disponibilité des produits |  | 02330288 |
| Sandoz | Sandoz Capsule January 2022 | Information Update | Mise à jour des renseignements |  | 0000 |
| Apotex | Manitoba Formulary Update: February 24, 2022 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | pms-AMLODIPINE 10mg | Product Transition | Transition dans la production |  | 02469049 |
| Teva | TEVA-TELMISARTAN HCTZ | Product Availability | Disponibilité des produits |  | 02330288 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Sandoz | Fer Suspension | Discontinuation | Abandon de produit | | 02246590 |
| Teva | OCTREOTIDE FOR INJECTABLE SUSPENSION | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Apotex | Newfoundland Price Change Update: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Sandoz | Sandoz Capsules December 2021 | Important Announcement | Annonce importante |  | 000 |
| Pharmascience | pms-PROGESTERONE 200mg GELCAPS BLI 2X15 | Product Availability | Disponibilité des produits |  | 02480247 |
| Pharmascience | pms-HYDROMORPHONE 1mg/mL SYRUP 500mL | Product Availability | Disponibilité des produits |  | 01916386 |
| Pharmascience | pms-PROGESTERONE 100mg GELCAPS BLI 2X15 | Product Availability | Disponibilité des produits |  | 02476576 |
| Apotex | Saskatchewan Formulary Update: Effective Immediately | Formulary Update | Mise à jour du formulaire |  | 0 |
| Teva | OCTREOTIDE FOR INJECTABLE SUSPENSION | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Teva | Loestrin | Information Update | Mise à jour des renseignements |  | 00353027 |
| Sandoz | Sandoz Capsule December 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsules December 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsules December 2021 | Important Announcement | Annonce importante |  | 0000 |
| Pharmascience | pms-ATORVASTATIN 80mg | Product Transition | Transition dans la production |  | 02477173 |
| Pharmascience | PHARMA-AMLODIPINE 2.5mg | Product Transition | Transition dans la production |  | 02469022 |
| Pharmascience | pms-ATORVASTATIN 80mg | Product Transition | Transition dans la production |  | 02477173 |
| Pharmascience | pms-AMOLDIPINE 5mg & 10mg | Product Transition | Transition dans la production |  | 02469030 & 02469049 |
| Teva | Octreotide for Injectable Suspension | New Product | Nouveau produit |  | 02503751, 02503778, 02503786 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Teva | Octreotide for Injectable Suspension | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Pharmascience | pms-Metformin 850mg TABS 100 | Product Transition | Transition dans la production |  | 02242589 |
| Apotex | Alberta Price Change: Effective December 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Saskatchewan Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | British Columbia Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Teva | OCTREOTIDE FOR INJECTABLE SUSPENSION | Formulary Update | Mise à jour du formulaire |  | 02503751, 02503778, 02503786 |
| Teva | OCTREOTIDE FOR INJECTABLE SUSPENSION | None Selected |  | 02503751, 02503778, 02503786 |
| Teva | Octreotide for Injectable Suspension | None Selected |  | 02503751, 02503778, 02503786 |
| Teva | Teva-Topilene | Information Update | Mise à jour des renseignements |  | 01927914 |
| Sandoz | Trandolapril | Product Appearance Up date | Nouvel aspect du produit |  | NA |
| Pharmascience | pms-DESMOPRESSIN 0.2mg | Product Availability | Disponibilité des produits |  | 02304376 |
| Apotex | Prince Edward Island Formulary Update: Effective November 22, 2021 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Prince Edward Island Price Change: Effective November 22, 2021 | None Selected |  | 0 |
| Pharma Science | pms-CELECOXIB 100mg CAPS 100 and 500 | Product Transition | Transition dans la production |  | 02355442 |
| Teva | TEVA-BETAMETHASONE/CALCIPOTRIOL | Formulary Update | Mise à jour du formulaire |  | 02427419, 02478307, 02478315, 02478323, 02478331, 02478358, 02466198 |
| Teva | Octreotide for Injectable Suspension | New Product | Nouveau produit |  | 02503751, 02503778, 02503786 |
| Apotex | Nova Scotia Price Change: Effective November 8, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Saskatchewan Price Change: Effective November 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Newfoundland Price Change: Effective November 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Manitoba Price Change: Effective November 25, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Mylan | FULPHILA NOW LISTED | Formulary Update | Mise à jour du formulaire |  | 02484153 |
| Pharmascience | Asaphen Chew 80mg | Product Transition | Transition dans la production |  | 02009013 |
| Pharmascience | pms-SILODOSIN | New Product | Nouveau produit |  | 02517779,02517787 |
| Pharmascience | pms-EVEROLIMUS | New Product | Nouveau produit |  | 02504677,02504685,02504693 |
| Apotex | Alberta Price Change: Effective November 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Pharmascience | MYINFLA ER 0.5mg | New Product | Nouveau produit |  | 02519380 |
| Teva | TEVA-BETAMETHASONE/CALCIPOTRIOL | Formulary Update | Mise à jour du formulaire |  | 02427419 |
| Teva | CEFAZOLIN SODIUM INJECTION | Formulary Update | Mise à jour du formulaire |  | 02108135 |
| Teva | Herzuma | New Product | Nouveau produit |  | 02480794, 02506211 |
| Teva | Teva-Teriparatide injectable | None Selected |  | 02486423 |
| Teva | Octreotide for Injectable Suspension | New Product | Nouveau produit |  | 02503751, 02503778, 02503786 |
| Sandoz | Sandoz Capsule October 2021 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule October 2021 | Important Announcement | Annonce importante |  | 0000 |
| Pharmascience | pms-NIFEDIPINE ER 30mg | Product Appearance Up date | Nouvel aspect du produit |  | 02418630 |
| Apotex | Apotex National Order Desk 2021 Holiday Hours | Information Update | Mise à jour des renseignements |  | 0 |
| Pharmascience | pms-LEVETIRACETAM | New Product | Nouveau produit |  | 02296101, 02296128,02296136 |
| Teva | Octreotide for Injectable Suspension | New Product | Nouveau produit |  | 02503751, 02503778, 02503786 |
| Teva | TEVA-IRBESARTAN HCTZ | Product Availability | Disponibilité des produits |  | 02330512, 02330520, 02330539 |
| Teva | TEVA-IRBESARTAN | Product Availability | Disponibilité des produits |  | 02316404, 02316412 |
| Teva | TEVA-BETAMETHASONE/CALCIPOTRIOL | Formulary Update | Mise à jour du formulaire |  | 02427419 |
| Teva | Teva-Teriparatide Injection | Formulary Update | Mise à jour du formulaire |  | 02486423 |
| Pharmascience | pms-ATOMOXETINE 10mg | Product Availability | Disponibilité des produits |  | 02381028 |
| Teva | Teva-Metoprolol Uncoated | Product Availability | Disponibilité des produits |  | 02261898 |
| Apotex | British Columbia Price Change: Effective October 21, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Nova Scotia Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Pharmascience | MYINFLA | New Product | Nouveau produit |  | 02519380 |
| Sandoz | TIMOLOL SDZ 0.5% 5ML EGEL CA | Product Availability | Disponibilité des produits |  | 02242276 |
| Sandoz | Dimethyl Fumarate Launch | New Product | Nouveau produit |  | 0000 |
| Pharmascience | pms-DIMETHYL FUMARATE | New Product | Nouveau produit |  | 02497026,02497034 |
| Apotex | National Price Change: Effective October 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Ontario Price Change: Effective September 30, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Manitoba Price Change: Effective October 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Newfoundland Price Change: Effective October 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Alberta Price Change: Effective October 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Ontario Price Change: Effective October 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | New Brunswick Price Change: Effective September 30, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Teva | Teva-Teriparatide Injection | Formulary Update | Mise à jour du formulaire |  | 02486423 |
| Teva | Irbesartan HCTZ | Product Availability | Disponibilité des produits |  | 02330512, 02330520, 02330539 |
| Teva | Irbesartan | Product Availability | Disponibilité des produits |  | 02316404, 02316412 |
| Teva | Metoprolol Uncoated | Product Availability | Disponibilité des produits |  | 02261898 |
| Pharmascience | pms-DESMOPRESSIN 0.1mg | Product Availability | Disponibilité des produits |  | 02304368 |
| Sandoz | Imipenem/Cilastatin for Injection USP 250/250MG | Discontinuation | Abandon de produit |  | 02358336 |
| Pharmascience | PMS-NITROFURANTOIN BID 100mg CAPS 100 | Product Appearance Up date | Nouvel aspect du produit |  | 02455676 |
| Pharmascience | pms-METHOTREXATE 2.5mg TABS BLI 3X10 | Price Update | Mise à jour des prix |  | 02170698 |
| Apotex | Nova Scotia Formulary Update: Effective September 6, 2021 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Saskatchewan Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Saskatchewan Formulary Update: Effective September 1, 2021 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | New Brunswick Formulary Update: Effective Immediately | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Saskatchewan Price Change: Effective September 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Sandoz | FER CF | Discontinuation | Abandon de produit |  | 02241802 |
| Sandoz | TIMOLOL SDZ 0.25% 5ML EGEL CA | Product Availability | Disponibilité des produits |  | 02242275 |
| Sandoz | Sandoz Capsule September 2021 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule September 2021 | Important Announcement | Annonce importante |  | 0000 |
| Teva | Teva-Dasatinib | Formulary Update | Mise à jour du formulaire |  | 02478307, 02478315, 02478323, 02478358 |
| Teva | Teva-Rosuvastatin | Product Transition | Transition dans la production |  | 02354608, 02354616, 02354624, 02354632 |
| Teva | Teva-Simvastatin, Teva-Tolterodine | Product Availability | Disponibilité des produits |  | 02299593, 02299607, 02250152 |
| Sandoz | TOBRAMYCIN INH | Discontinuation | Abandon de produit |  | 02443368 |
| Teva | Teva-Tolterodine; Teva-Simvastatin | Product Availability | Disponibilité des produits |  | 02299593, 02299607, 02250152 |
| Teva | Teva-Rosuvastatin | Product Transition | Transition dans la production |  | 02354608, 02354616, 02354624, 02354632 |
| Apotex | British Columbia Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Ontario Formulary Update: Effective August 31, 2021 | Formulary Update | Mise à jour du formulaire |  | 0 |
| Apotex | Ontario Price Change: Effective August 31, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Sandoz | Sandoz Capsule August 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule August 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule of August 2021 | Important Announcement | Annonce importante |  | 000 |
| Teva | Teva-Dasatinib | Formulary Update | Mise à jour du formulaire | | 02478307, 02478315, 02478323, 02478358 |
| Sandoz | Ci-Cal D | Discontinuation | Abandon de produit |  | 80030816 |
| Apotex | Saskatchewan Price Change: Effective August 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | New Brunswick Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | New Brunswick Price Change: Effective July 29, 2021 | None Selected |  | 0 |
| Apotex | pms-ESCITALOPRAM | New Product | Nouveau produit | 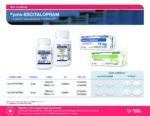 | 02469243,02469251 |
| Teva | Teva-Rosuvastatin | Product Transition | Transition dans la production |  | 02354608, 02354616, 02354624, 02354632 |
| Teva | Teva-Tolterodine | Product Availability | Disponibilité des produits |  | 02299593, 02299607 |
| Sandoz | DICLOFENAC SR 75MG | Discontinuation | Abandon de produit |  | 2261901 |
| Sandoz | Cyproheptadine | Discontinuation | Abandon de produit |  | 02245668 |
| Apotex | pms-METOCLOPRAMIDE HYDROCHLORIDE INJECTION | New Product | Nouveau produit |  | 02510790 |
| Teva | TEVA-BETAMETHASONE/CALCIPOTRIOL | Formulary Update | Mise à jour du formulaire |  | 02427419 |
| Mylan | FULPHILA CODIFICATION NOTICE | Information Update | Mise à jour des renseignements |  | 02484153 |
| Sandoz | Sandoz Capsule July 2021 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz capsule July 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule July 2021 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Orphenadrine | Product Availability | Disponibilité des produits |  | 02243559 |
| Teva | Act Rizatriptan | Product Transition | Transition dans la production |  | 02381702 |
| Teva | Teva- Cephalexin Susp, Teva-Anastrozole, Teva-Zopiclone, Teva-Bupropion XL, Teva-Buprenorphine/Naloxone, Teva-Cephalexin | Product Appearance Up date | Nouvel aspect du produit |  | 00342092, 02394898, 02242481, 02439654, 02439662, 02453908, 00342084 |
| Teva | Teva-Buprenorphine/Naloxone, Teva-Cephalexin | Product Appearance Up date | Nouvel aspect du produit |  | 02453908, 00342084 |
| Teva | Act Rizatriptan | Product Transition | Transition dans la production |  | 02381702 |
| Pharmascience | pms-METHOTREXATE 2.5mg BLI 3X10 | New pack size | Nouveau format |  | 02170698 |
| Apotex | Apobiologix® Quebec Price Change: Lapelga® 6 mg/0.6 mL Sterile Solution for Injection | Price Update | Mise à jour des prix |  | 02474565 |
| Apotex | Newfoundland Price Change: Effective July 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | New Brunswick Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Saskatchewan Price Change: Effective July 1, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | British Columbia Price Change: Effective July 15, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Nova Scotia Price Change: Effective July 12, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Teva | Teva-Dasatinib | Formulary Update | Mise à jour du formulaire |  | 02478307, 02478315, 02478323, 02478331, 02478358 |
| Teva | | None Selected | | 02466198 |
| Pharmascience | pms-AMLODIPINE | Product Appearance Up date | Nouvel aspect du produit |  | 02295148, 02284065,02284073 |
| Apotex | Prince Edward Island Price Change: Effective June 28, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Apotex | Newfoundland Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Apotex | NIHB Formulary Update: Effective Immediately | Formulary Update | Mise à jour du formulaire |  | 0 |
| Teva | Teva-Dasatinib, Teva-Febuxostat | Formulary Update | Mise à jour du formulaire |  | 02478307, 02478315, 02478323, 02478358, 02466198 |
| Sandoz | Sandoz Capsule June 2021 | Important Announcement | Annonce importante |  | 0000 |
| Sandoz | Sandoz Capsule June 2021 | None Selected |  | 0000 |
| Sandoz | Sandoz Capsule June 2021 | Important Announcement | Annonce importante |  | 000 |
| Sandoz | Sandoz Capsule June 2021 | Important Announcement | Annonce importante |  | 000 |
| Apotex | Ontario Price Change: Effective June 30, 2021 | Price Update | Mise à jour des prix |  | 0 |
| Teva | Teva-Dasatinib, Teva-Febuxostat | Formulary Update | Mise à jour du formulaire |  | 02478307, 02478315, 02478323, 02478331, 02478358, 02466198 |
| Teva | Teva-Zopiclone, Teva Bupropion XL | Product Appearance Up date | Nouvel aspect du produit |  | 02242481, 02439654, 02439662 |
| Teva | ACT Rizatriptan | Product Transition | Transition dans la production |  | 02381702 |
| Teva | Teva-Pindolol, Teva-Zopiclone | Product Availability | Disponibilité des produits |  | 00869007, 00869015, 02246534, 02242481 |
| Mylan | FULPHILA PRICE CHANGE | Price Update | Mise à jour des prix |  | 02484153 |
| Apotex | British Columbia Price Changes | Price Update | Mise à jour des prix |  | 0 |
| Apotex | New Brunswick Price Change: Effective Immediately | Price Update | Mise à jour des prix |  | 0 |
| Teva | Teva-Pindolol, Teva-Zopiclone | Product Availability | Disponibilité des produits |  | 00869007, 00869015, 02246534, 02242481 |
| Teva | Teva-Rosuvastatin | Product Transition | Transition dans la production |  | 02354608, 02354624, 02354632 |
| Teva | Teva-Febuxostat, Teva-Nabilone | Formulary Update | Mise à jour du formulaire |  | 02466198, 02392925 |
| Teva | Teva-Dasatinib, Teva-Febuxostat | Formulary Update | Mise à jour du formulaire |  | 02478307, 02478315, 02478323, 02478358, 02466198 |
| Teva | TEVA-TOPISONE | Product Appearance Update | Missing Attachment | 809187 |
| Apotex | Apo-Lansoprazole-Amoxicillin-Clarithromycin 30 mg DR Caps, 500 mg Caps, 500 mg Tabs | Formulary Update |  | 2,470,780 |
| Apotex | Product Discontinuation: September 27, 2018 | Discontinuation |  | 0 |
| Apotex | Product Pack Discontinuation: September 20, 2018 | Discontinuation |  | 0 |
| Teva | Methoxisal - Methoxacet | Discontinuation | Missing Attachment | 2,236,872,019,663,750,000,000,000,000,000 |
| Teva | Allernix | Discontinuation | Missing Attachment | 209,758,302,097,575 |
| Sandoz | Sandoz Lacosamide | New Product |  | 2,474,670,024,746,890,000,000,000,000,000 |
| Teva | Teva-Lacosamide | New Product | Missing Attachment | 2,472,902,024,729,100,000,000,000,000,000 |
| Apotex | Apo-Emtricitabine-Tenofovir 200/300 mg Tabs | Price Update |  | 2,452,006 |
| Teva | ACT QUETIAPINE | Product Transition | Missing Attachment | 231,608,002,316,099,000,000,000,000,000,000,000,000 |
| Teva | ACT PIOGLITAZONE | Product Transition | Missing Attachment | 230,286,102,302,888,000,000,000,000,000,000,000,000 |
| Teva | Teva-Trandolapril | New Product | Missing Attachment | 2,415,429,024,154,370,000,000,000,000,000 |
| Teva | TEVA-TOLTERODINE LA | Formulary Update | Missing Attachment | 241,219,502,412,209 |
| Teva | TEVA-SOLIFENACIN | Formulary Update | Missing Attachment | 239,790,002,397,919 |
| Teva | TEVA-ARIPIPRAZOLE | Formulary Update | Missing Attachment | 246,417,902,464,179 |
| Sandoz | Sandoz Esomeprazole | New Product |  | 246,092,002,460,939 |
| Mylan | Pyridoxine Hydrochloride Injection | Important Announcement | | 2,245,215 |
| Sandoz | Haloperidol LA | Product Availability |  | 9,999,999 |
| Sandoz | Haloperidol LA | Product Availability |  | 9,999,999 |
| Apotex | Apo-Pinaverium 50 mg & 100 mg tablets | New Product |  | 246,967,702,469,685 |
| Sandoz | Sandoz Capsule at a Glance | Formulary Update |  | 24,687,000,246,871,900,000,000 |
| Apotex | Apo-Lansoprazole-Amoxicillin-Clarithromycin 30 mg DR Caps, 500 mg Caps, 500 mg Tabs | Formulary Update |  | 2470780 |
| Apotex | British Columbia Price Change: Effective September 11, 2018 | Price Update |  | 0 |
| Teva | Teva-Aripiprazole | New Product | Missing Attachment | 246,414,402,464,152,000,000,000,000,000,000,000,000 |
| Apotex | British Columbia Formulary Update: September 7, 2018 | Formulary Update |  | 0 |
| Apotex | Apo-Efavirenz-Emtricitabine-Tenofovir 600 mg / 200 mg / 300 mg Tabs | New Product |  | 2,468,247 |
| Teva | TEVA-ARIPIPRAZOLE | Formulary Update | Missing Attachment | 246,417,902,464,187 |
| Apotex | Apo-Solifenacin 5 mg & 10 mg Tabs | New Product |  | 242,337,502,423,383 |
| Apotex | Apo-Metronidazole 500 mg Caps | Price Update |  | 2,248,562 |
| Sandoz | Sandoz Ranitidine | Product Appearance Update |  | 224,322,902,243,230 |




































































































































































































































































































































































